Epoch Master Global Business(Jiangsu)Inc.
Address:Rm.3-93,Tengfei building,No.88 jiangmiao rd., research and innovation park,
Nanjing zone,(jiangsu) pilot free trade zone,China
Tel:13770711448 Email:sales01@epoch-master.com
1.1 Classification
Phosphates can be divided into orthophosphates and condensed phosphates:
Orthophosphate refers to various salts of orthophosphoric acid (H3PO4): M3PO4, M2HPO4, MH2PO4 (M is a monovalent metal ion).
Orthophosphate is heated, dehydrated and condensed to form condensed phosphate. Its general formula is Mn+2PnO3n+1, where M is a monovalent metal ion and n is the number of phosphorus atoms. When the value of n is very large, the limiting chemical formula of condensed phosphate is: MnPnO3n.
The various salts of pyrophosphate are called pyrophosphates, M4P2O7;
The various salts of triphosphate are called tripolyphosphates, M5P3O10;
Condensed phosphates whose molecules contain more than 3 phosphorus atoms are collectively called polyphosphates, and the number of O-P-O bonds in their molecules is called the chain length of polyphosphates.
The molecular formula of metaphosphate is (MPO3)n, which can be roughly divided into cyclic metaphosphate, insoluble metaphosphate and metaphosphate vitreous (such substances are actually chain polyphosphates with a chain length of more than 10 and a small amount of mixture of cyclic metaphosphates).
1.2 The phosphates used in food processing are usually sodium salts, calcium salts, potassium salts, and iron salts and zinc salts as nutritional fortifiers. There are more than 30 types of commonly used food-grade phosphates. Sodium phosphate is At present, the main consumption category of domestic food phosphate is potassium phosphate. With the development of food processing technology, the consumption of potassium phosphate is also increasing year by year.
In order to give full play to the synergistic effects between various phosphates and phosphates and other additives, and meet the development needs of food processing technology, various compound phosphates are often used as food ingredients and functional additives in practical applications. The research and development of formulated phosphates has increasingly become the development direction for the development and application of phosphate food additives.
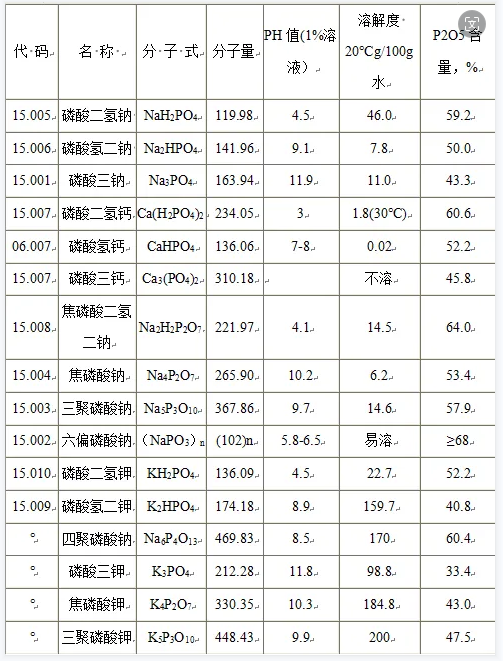
Due to differences in chain length, pH value, P2O5 content and bound metal cations, different types of phosphates have great differences in physical and chemical properties. For linear polyphosphates, as the chain length increases, its emulsification, dispersion properties and ability to chelate calcium ions increase, while the buffering effect and pH value decrease.
Condensed polyphosphate will hydrolyze under heating or acidic conditions to generate orthophosphate or short-chain polyphosphate. When there are enzymes, gels, and complex cations in the solution, the hydrolysis speed can be greatly accelerated, and the solution's As the ionic strength increases, the hydrolysis rate can be accelerated several times.
In practical applications, phosphates are often selected rationally according to the requirements of food processing technology and based on their pH value and buffering effect, solubility, water-holding effect, emulsification, dispersion performance, chelation, hydrolysis stability and other characteristics. As food ingredients and functional additives.
2.2 Characteristics of phosphates and their role in food processing
Phosphate has two main functions in food processing: first, as a quality improver to improve the structure and taste of food; second, it can be used as a mineral nutrition fortifier.
The role of phosphate in food processing is mainly based on the following properties of phosphate:
2.1 Buffering effect:
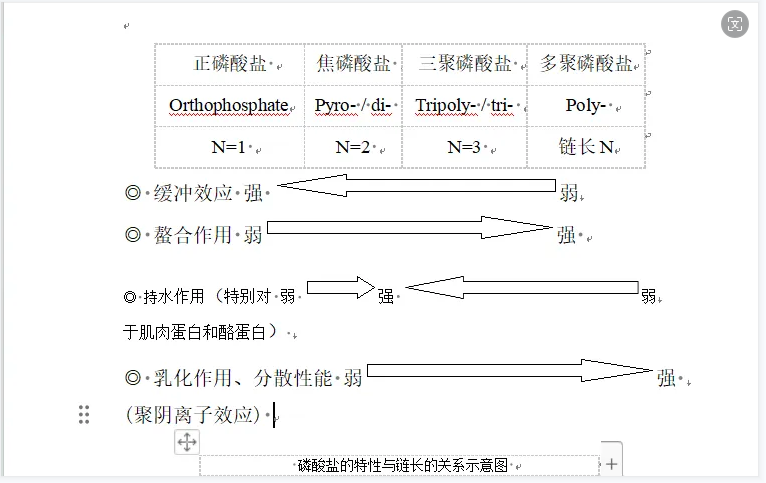
The pH value of phosphate ranges from moderately acidic (PH~4) to strongly alkaline (PH~12). When different phosphates are combined in different proportions, the pH value can be stabilized between PH4.5-11.7. level of buffer. Within the pH range of most foods (PH 3.5-7.5), phosphate can be used as an efficient pH regulator and pH stabilizer to make food taste more delicious. The strongest buffering effect is orthophosphate. For polyphosphate, as the chain length increases, the buffering capacity will weaken.
2.2 Water holding effect:
Polyphosphate is a highly hydrophilic moisture retaining agent that can stabilize the moisture contained in food. The quality of its water-holding capacity is related to factors such as the type and amount of polyphosphate, the PH value of the food, and the ionic strength.
For meat products and seafood, pyrophosphate has the best water-holding capacity, followed by tripolyphosphate. As the chain length increases, the water-holding capacity of polyphosphate will weaken.
2.3 Polyanion effect:
Polyphosphate is a polymeric dielectric and has the characteristics of an inorganic surfactant. It can disperse insoluble substances in water or form a stable suspension to prevent the adhesion and agglomeration of the suspension. Because polyphosphate can make the protein hydrosol form a film on the fat globules, thereby dispersing fat more effectively in water, it is widely used in the phosphorylation of starch, the dispersion of pigments, and emulsified foods (milk products, ice cream, salads, sauces, etc.) and used as a dispersion stabilizer for sausages, minced meat products, and surimi products.
For linear polyphosphates, their emulsifying and dispersing abilities increase as the chain length increases.
2.4 Chelation:
Polyphosphate easily forms soluble complexes with metal cations in the solution, thereby reducing the hardness of water, inhibiting the oxidation, catalysis, discoloration, and decomposition of vitamin C caused by metal cations such as Cu2+ and Fe3+ to prevent and delay fat Oxidation, prevent meat, poultry, fish from spoilage, maintain color and extend the shelf life of food. Its boiling ability is shown in the figure below
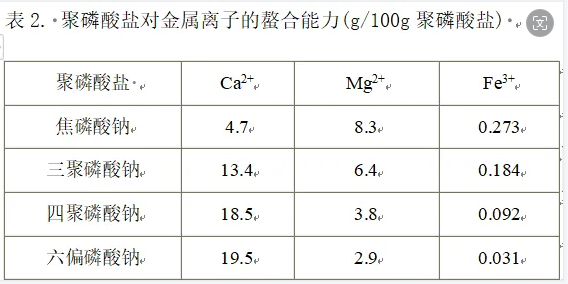
The chelating effect of polyphosphates depends on chain length and pH. Generally speaking, long-chain polyphosphates have strong chelating ability for light metal ions, which increases with the increase of pH value; short-chain polyphosphates have strong chelating ability for heavy metal ions, but the ability to chelate heavy metal ions increases with the increase of pH value. As the level increases, the chelation effect weakens.
2.5 Protein function:
Phosphate has a strengthening effect on protein and collagen globulin, so it can improve the hydration and water-holding capacity of meat products, increase water permeability, promote the softening of food, improve the quality of food, and maintain the flavor of food. At the same time, phosphates in dairy products can prevent the coagulation of milk when heated and prevent the separation of casein and fat moisture.
2.6 Bulking effect:
Acidic phosphates (such as sodium acid pyrophosphate, calcium hydrogen phosphate) are usually used as leavening acids as leavening agents for baked products, and react with bicarbonate to provide the carbon dioxide gas required for the baking process.
2.7 Anti-caking effect:
Tricalcium phosphate is commonly used as an anti-caking agent to improve the free-flowing properties of powdered or hygroscopic foods.
Tricalcium phosphate has a larger specific surface area and can bind more water; and its special spherical crystal structure can produce a "ball effect", giving the powder good free-flowing properties.
2.8 Extend the shelf life of food:
Polyphosphate can enhance the storage stability of food and extend the shelf life of products. This effect is mainly based on: (1) pH regulation; (2) Antibacterial effect: Microbial cell growth must rely on divalent metal cations, especially Ca2+ and Mg2+, and phosphate can chelate with these metal cations, and it It can reduce the stability of the cell wall during cell division and also reduce the thermal stability of many cells, thereby effectively inhibiting bacterial growth.
The antibacterial effect of polyphosphate is related to its type (chain length), content, pH value, salt content, nitrite content and other factors. Generally speaking, as the chain length increases, the antibacterial effect increases.
2.9 Mineral nutrition strengthening effect:
Calcium phosphate, magnesium phosphate, iron phosphate, and zinc phosphate are often used as mineral enhancers in food processing.
Adding iron phosphate and zinc phosphate to gastric juice can enhance the biopharmaceutical effect of gastric juice due to its better solubility and will not promote natural oxidation.
The safety of phosphates used as food additives is a matter of great concern. Many foreign scientists have conducted a large number of toxicological studies on phosphates and confirmed that food phosphates are non-toxic and highly safe additives.
The safety evaluation of the Special Committee of the Food and Agriculture Organization of the United Nations and the World Health Organization (FAO/WHO) in 1970 was that the daily allowable intake of adults is 1.4-1.5gP2O5, while the Food Additives Committee in 1985 recommended that the unconditional acceptance of total phosphorus in the diet is <30 mg/kg body weight, conditional acceptance dose is 30--70 mg/kg body weight.
It must be pointed out here that in the application of food phosphates, attention should be paid to the balance of calcium and phosphorus (the calcium to phosphorus ratio is 1:1.2), and food phosphates should be used rationally in strict accordance with the hygienic standards for the use of food additives. , to avoid adverse effects on human health caused by calcium and phosphorus imbalance or abuse of phosphates.
4.1 Application in meat and poultry product processing:
4.1.1 In order to improve the quality of meat products, phosphate is usually added to the processing of meat products. Its functions are:
a. Improve the adhesion of meat products and improve the slicing performance of meat products;
b. Improve the water-holding capacity of meat, so that meat products can still maintain their natural moisture during processing and cooking, reduce the loss of meat nutrients, preserve the tenderness of meat products, and increase the yield of finished products;
c. Control the pH value of meat products in the range most suitable for protein swelling and produce the best color of meat products;
d. Improve emulsification performance and emulsion stability, effectively preventing the separation of fat and water;
e. Block metal cations and delay the oxidation reaction in the processing of meat products, which can effectively reduce the rancidity rate of products, inhibit the discoloration and rancidity of meat products, and extend the shelf life of meat products;
f. Improve the processing performance of meat products and increase production efficiency.
4.1.2 The water holding capacity of meat generally refers to the ability to retain the moisture of meat and the moisture added to the meat during processing. The level of water holding capacity is directly related to the texture and yield of meat products. Adding phosphate can effectively improve the quality of meat products. Water holding capacity.
How to rationally use phosphates and other additives without affecting the flavor of meat products, maximize the water holding capacity and cohesion of meat products, and reduce the cooking loss of meat products has always been an important topic in the research and development of meat products. .
4.1.3 Reasonable use of phosphates in meat product processing:
In practical applications, the appropriate type and amount of phosphate should be selected based on the type, texture requirements, production process, raw materials, etc. of the meat products and the characteristics of various phosphates.
Meat products added with pyrophosphate can restore and enhance the natural water-holding capacity of muscle protein. Polyphosphate can be quickly converted into pyrophosphate under the action of muscle enzymes, so the same effect can be achieved. Although pyrophosphate has the best water retention effect, its solubility is too poor, so it cannot be used alone in most cases. Instead, it is often used in combination with long-chain polyphosphate or potassium phosphate with better solubility. In addition, in order to exert the synergistic effect between various phosphates and phosphates and other additives, various compound meat product improvers are often used.
The functions of various compound meat product phosphates are described as follows:
a. For sausages and minced meat products, pyrophosphate and medium chain length polyphosphate are usually used, which are added in the form of dry powder during chopping and mixing. The pH value of the complex phosphate used is generally around 7, and sometimes complex phosphates with a pH value higher than 9 are also used.
b. The compound phosphate used for injection of saline must meet the following requirements: 1) good solubility in icy saline; 2) high dissolution rate; 3) good stability in icy saline. The pH value of the complex phosphate used is generally 8.5--9.5. In order to achieve the best muscle protein activation effect when preparing iced saline for injection, it is best to dissolve the phosphate in iced water first, and then add salt. This order generally cannot be reversed.
c. The amount of mixed phosphate added is generally 0.1-0.4%, but the amount should be strictly controlled when used. If the amount added is too high, the original flavor of the meat will be damaged, and the color development will be affected due to the increase in pH value.
4.2 Application in seafood processing:
4.2.1 As an excellent water-retaining agent, pH regulator and antifreeze agent, phosphate is widely used in the processing of seafood, especially frozen seafood. Its functions are:
a. Effectively improve the water-holding capacity of seafood, make the meat juice richer, and effectively retain nutrients and moisture;
b. Inhibit fat oxidation and effectively extend the shelf life of seafood;
c. Reduce dripping loss after thawing and reduce cooking weight loss;
d. Maintain the natural color and flavor of seafood;
e. Synergizes with sugar to effectively prevent freezing denaturation of surimi protein.
4.2.2 When processing frozen shrimp, fish, and shellfish seafood, the products are usually soaked in 3~10% compound phosphate solution (the temperature is less than 10°C). The concentration and soaking time of the soaking solution are based on the shrimp, fish, and shellfish Determined by the type, size and fishing time of seafood.
The following factors should be considered when rationally selecting compound phosphates for soaking: a) It can effectively improve the water-holding capacity of seafood; b) It has good solubility in ice water; c) It can dissolve quickly in ice water; d) It has good solubility in ice water. Good stability. The pH value of the complex phosphate used is generally higher than 9.
4.2.3 Generally, the compound phosphates added to frozen surimi are mainly sodium pyrophosphate, sodium tripolyphosphate and sodium hexametaphosphate, and the added amount is 0.1-0.3% of surimi.
4.3 Application of phosphate in flour products
4.3.1 Application in baked products:
Acidic phosphates (such as sodium acid pyrophosphate, calcium hydrogen phosphate) are usually used as leavening acids as leavening agents for baked products, and react with bicarbonate to provide the carbon dioxide gas required for the baking process. Different phosphates have different dough reaction rates (ROR), and phosphates can be reasonably selected based on the expected baking effect (loose volume, pore structure, taste).
In addition, phosphates can also be used as flour conditioners, dough improvers, buffers and yeast nutrients.
4.3.2 As a noodle quality improver, phosphate is widely used in the processing of instant noodles and ordinary noodles. Its main functions are:
a. Increase the degree of starch gelatinization, increase the water absorption capacity of starch, increase the water holding capacity of the dough, and make instant noodles rehydrate quickly and brew easily;
b. Enhance the water-absorbing swelling performance of gluten protein and improve its elasticity, making the noodles smooth and chewy, and resistant to cooking and soaking;
c. The excellent buffering effect of phosphate can stabilize the PH value of the dough, prevent discoloration and deterioration, and improve the flavor and taste;
d. Phosphate can complex with metal cations in the dough, and has a "bridging" effect on the glucose groups, forming cross-links of starch molecules, so that noodles that can withstand high-temperature cooking and high-temperature frying can still maintain their consistency after rehydration. Viscoelastic characteristics of starch colloids;
e. Improve the finish of noodles;
4.4 Application in dairy products
Phosphate is used as a stabilizer and emulsifier in UHT-sterilized milk, cream products, condensed milk, milk powder, coffee mate, milk drinks, and cheese products. Its functions are:
a. Buffering and pH stabilization;
b. Interaction with protein: disperses food ingredients, stabilizes the emulsification system, enhances casein's ability to bind water, and effectively prevents the separation of protein, fat and water;
c. Chelate multivalent metal ions, greatly reducing protein aggregation and precipitation during heating and storage, thus improving the thermal stability and storage stability of milk. And it can effectively delay the occurrence of lactose coagulation.
4.5 Phosphate is also widely used in the following food processing fields:
◎ Beverages: used as acidity regulator, stabilizer and mineral nutrition fortifier;
◎ Potato products: used as stabilizers and color-preserving agents;
◎ Rice products: improve the elasticity of products and improve the taste of products;
◎ Seasonings and instant soups: stabilizers, acidity regulators;
◎ Hygroscopic powdered food: prevent caking and improve its free-flowing performance;
◎ Starch products and modified starch;
◎Baby food, functional food: mineral nutrition fortifier.